Peter Bøggild, Department of Micro- and Nanotechnology and in the Center for Nanostructured Graphene , Printed in Nature International Journal of Science
The material graphene has a vast number of potential applications — but a survey of commercially available graphene samples reveals that research could be undermined by the poor quality of the available material.
Graphite is composed of layers of carbon atoms just a single atom in thickness, known as graphene sheets, to which it owes many of its remarkable properties. When the thickness of graphite flakes is reduced to just a few graphene layers, some of the material’s technologically most important characteristics are greatly enhanced — such as the total surface area per gram, and the mechanical flexibility of the individual flakes. In other words, graphene is more than just thin graphite. Unfortunately, it seems that many graphene producers either do not know or do not care about this. Writing in Advanced Materials, Kauling et al. report a systematic study of graphene from 60 producers, and find that many highly priced graphene products consist mostly of graphite powder.
Imagine a world in which antibiotics could be sold by anybody, and were not subject to quality standards and regulations. Many people would be afraid to use them because of the potential side effects, or because they had no faith that they would work, with potentially fatal consequences. For emerging nanomaterials such as graphene, a lack of standards is creating a situation that, although not deadly, is similarly unacceptable.
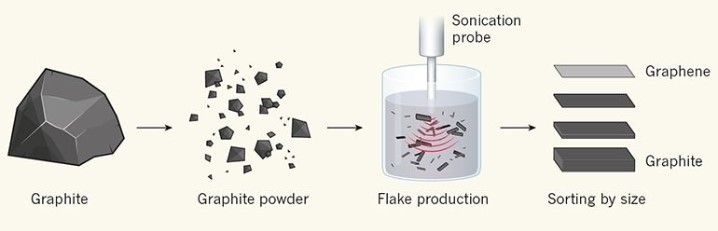
One of the most well-established methods for producing graphene for commercial applications is liquid-phase exfoliation (LPE) — a process that involves milling graphite into a powder, and separating the particles into tiny flakes by applying mechanical forces in a liquid. Those precious flakes that contain just a few layers of graphene are then separated from the rest (Fig. 1). Graphene produced in this way has a huge number of potential applications, including battery technology, composite materials and solar cells. The LPE of graphite was first achieved using sonication to produce the flakes, and later work showed that even a kitchen blender can be used to create violent turbulent forces that pull graphene sheets apart without destroying them.
Most commercially available bulk graphene is made by milling graphite into powder, and then subjecting the resulting particles to mechanical forces in a liquid solution to separate the powder into flakes, for example, by using sonication; flakes not shown to scale. The flakes are then sorted according to their size and thickness. Kauling et al. analysed commercially available graphene from 60 providers, and found that the majority of the samples contained less than 10% of graphene (flakes that contain fewer than ten layers of carbon atoms). The rest is essentially just graphite powder. (Adapted from ref. 1.)
But how thin must graphite flakes be to behave as graphene? A common idea, backed up by the International Organization for Standardization (ISO), is that flakes containing more than ten graphene layers are basically graphite. This seemingly arbitrary threshold has some basis in physics, as Kauling et al. note. For example, thermodynamic considerations dictate that each layer of atoms in a flake of ten or fewer layers behaves as an individual graphene crystal at room temperature. Moreover, the rigidity of flakes scales with the cube of layer thickness, which means that thin graphene flakes are orders of magnitude more flexible than thicker graphite flakes.
So size really matters: depending on the practical application, graphene and graphite powders can give entirely different results. Without clear standards by which to determine the quality of commercially available graphene, companies and researchers risk wasting time and money doing research on graphite powder disguised as expensive, high-grade graphene. This would stunt the development of graphene technology, harming serious graphene producers and application developers alike.
But are these concerns truly warranted? In a study aimed at answering this question, Kauling et al. established a systematic test protocol based on an arsenal of well-established methods for characterizing graphene, and then used the protocol to benchmark 60 graphene products from different producers, a daunting task. The results showed that the statistical distributions of the key material indicators — such as the size, structural integrity and purity of the graphene — varied greatly. Shockingly, the study revealed that less than 10% of the material in most of the products consisted of graphene composed of ten or fewer layers. None of the products tested contained more than 50% of such graphene, and many were heavily contaminated, most likely with chemicals used in the production process.
It seems that the high-profile scientific discoveries, technical breakthroughs and heavy investment in graphene have created a Wild West for business opportunists: the study shows that some producers are labelling black powders that mostly contain cheap graphite as graphene, and selling them for top dollar. The problem is exacerbated because the entry barrier to becoming a graphene provider is exceptionally low — anyone can buy bulk graphite, grind it to powder and make a website to sell it on.
Unless common standards and test protocols are introduced, there is a great risk of dropping the ball at the worst possible time. Dozens of emerging applications for graphene are closely linked to some of society’s grand challenges: health, climate, renewable energy and sustainability. Some of these applications might never leave the starting block if the early development is based on ‘fake graphene’.
Kauling and colleagues’ article is therefore a much-needed wake-up call for graphene producers, buyers and researchers to agree on and to adhere to sound standards: a transparent graphene market would benefit everyone, except perhaps unscrupulous vendors. The first steps towards this have already been taken with the ISO’s graphene vocabulary (a document that defines standard terminology for describing graphene) and the UK National Physical Laboratory’s helpful Good Practice Guide for graphene characterization. Now it’s time to push on.
It should be noted that Kauling and co-workers’ study does not cover all the types of bulk graphene on the market. Moreover, although the authors analysed an impressive number of LPE-manufactured products, they could have eliminated any accusations of potential bias by specifying the criteria they used to select the products for analysis. It is also possible that they unintentionally missed high-quality graphene sold by a few excellent producers. And, as the researchers mention, different applications generally make use of different characteristics of graphene — which makes it difficult to come up with a universal metric of quality.
Nevertheless, the work is a timely and ambitious example of the rigorous mindset needed to make rapid progress, not just in graphene research, but in work on any nanomaterial entering the market. To put it bluntly, there can be no quality without quality control.